Your message has been sent.
 CLOSE SIDEBAR
CLOSE SIDEBAR
The Effects of Mechanical Tissue Resuscitation (MTR) for Treatment of Ischemia Reperfusion Injury in a Swine Model of Acute Myocardial Infarction
Nicholas A. Mouser, B.S., Magan Lane, B.S., James E. Jordan, Ph.D.
Poster Title: The Effects of Mechanical Tissue Resuscitation (MTR) for Treatment of Ischemia Reperfusion Injury in a Swine Model of Acute Myocardial Infarction
Student: Nicholas Mouser, Class of 2024
Faculty Mentor and Department: James E. Jordan, PhD, Department of Cardiothoracic Surgery
Funding Source: Groskort (Lois I.) Heart Research
ABSTRACT
Background: Heart disease is the leading cause of death in the US and worldwide. For those who suffer from heart attack, reperfusion of ischemic cardiac tissue is the primary objective. While necessary, reperfusion promotes various inflammatory processes that may ultimately lead to cardiomyocyte injury and death, otherwise called ischemia-reperfusion injury (IRI). Modern pharmacological interventions attempt to reduce the extent of damage by targeting specific and selective biochemical pathways. Yet due to the broad spectrum of mediators that contribute to IRI, these therapies have proven unsuccessful. Presently, no treatment effectively attenuates IRI. Mechanobiology is an alternative intervention that involves physical manipulation of living tissues to elicit a biological change. Specifically, prior research directed toward reducing IRI has demonstrated that application of a uniform, negatively pressurized vacuum to ischemic tissue, or area at risk (AAR), mitigates inflammatory processes and the amount of necrosis within the AAR. This study aims to find the most effective length of MTR treatment – 60min (MTR-60), 120min (MTR-120), or 180min (MTR-180) – at a pressure of -125mmHg.
Hypothesis: Preliminary work with MTR has indicated that moderation is necessary for effective treatment. In other words, balancing vacuum pressure and duration of treatment is essential for reducing IRI. For this reason, it is hypothesized that MTR-120 will provide the best therapeutic effect.
Methods: A cohort of 34 Yorkshire swine were randomized to one of four groups: control (no treatment), MTR-60, MTR-120, or MTR-180. Each animal was placed under general anesthesia before instrumentation. Pressure catheters were inserted into the right femoral artery and left femoral artery for pressure monitoring. The right external jugular was isolated, and a catheter was placed for fluid administration. Next, a sternotomy was performed, and monofilament sutures were placed, but not secured, around several branches of the LAD. The left atrium was canulated for microsphere administration to allow for measurements of regional myocardial blood flow. Ischemia was induced by securing rubber tourniquets around the selected LAD branches. The tourniquets were removed to allow for reperfusion after 70min, and the MTR device was applied to the AAR for the designated time. Hemodynamic, blood gas, and blood flow data were collected at regular time intervals throughout the five-hour study. After completion of the reperfusion phase, differential staining of the heart was performed to discriminate ischemic from non-ischemic tissue. The animal was then euthanized, the heart extracted, and further staining completed to help quantify non-necrotic ischemic tissue from necrotic tissue.
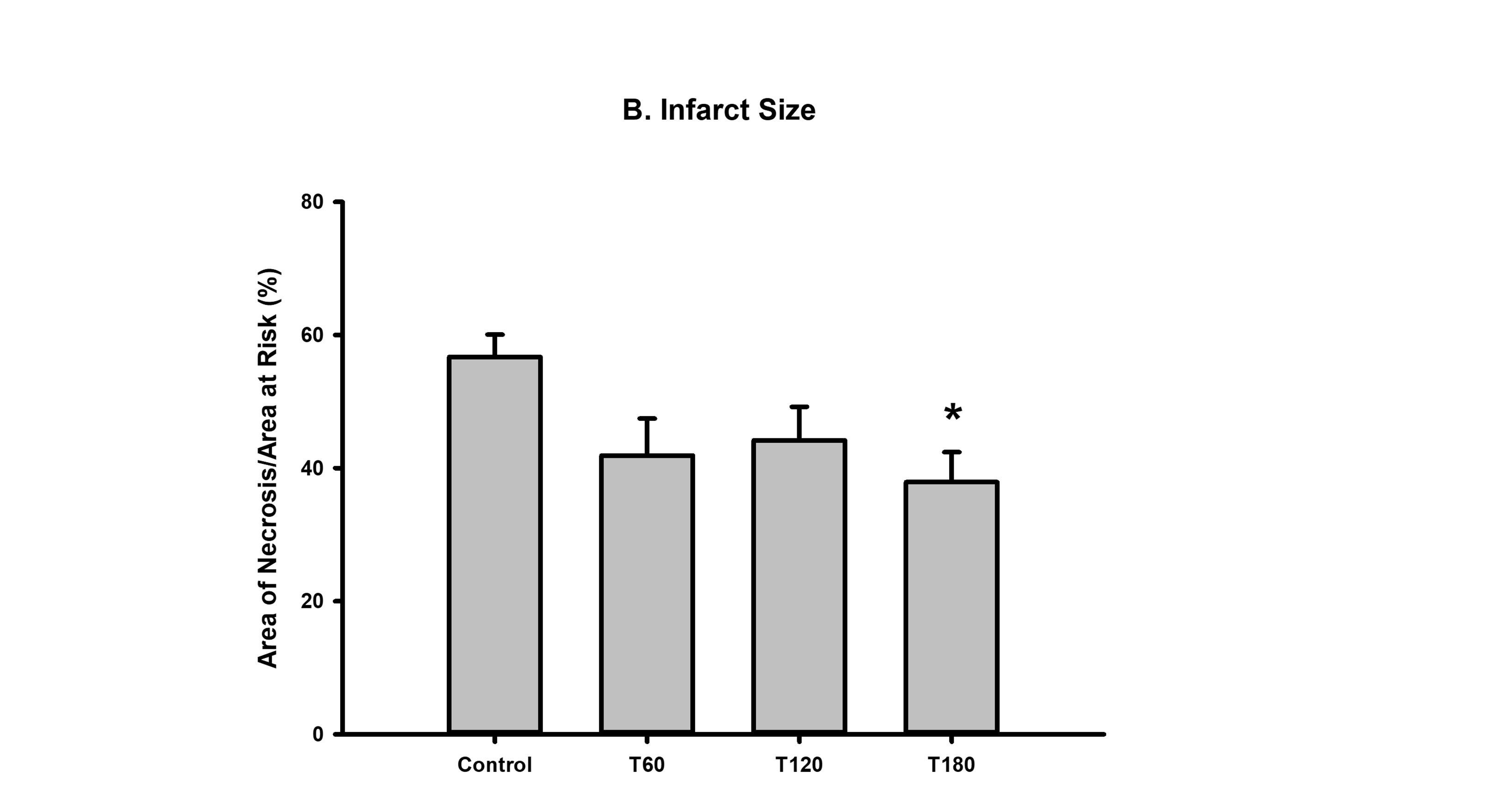
Results: There were no statistically significant differences in the AAR among the control group, MTR-60, MTR-120, and MTR-180 (16.86±2.0%, 15.86±1.9%, 16.40±1.2%,14.29±1.6% respectively). MTR-60 (41.90±16.7%) and MTR-120 (44.20±14.2%) reduced the AN relative to the control (56.72±10.1), though neither group reached statistical significance. However, infarct size was significantly reduced in MTR-180 (37.90±12.76; p<0.05).
Conclusions: Based on these findings, MTR-180 is the most effective option for reducing infarct size in the setting of IRI. This suggests that longer treatment times are necessary to obtain optimal benefits. It is suspected that over the course of reperfusion, the sustained vacuum helps clear edema and eliminate inflammatory mediators. While these results support MTR as a viable treatment for IRI, it will be crucial to perform survival studies in the future to further characterize long term therapeutic benefits of MTR.
Source of mentor’s funding or other support that funded this research: Cheek Foundation Endowment of the Department of Plastic & Reconstructive Surgery, Wake Forest Department of Cardiothoracic Surgery
Powered by Acadiate
© 2011-2024, Acadiate Inc. or its affiliates · Privacy
